Resources
SELECT trial information
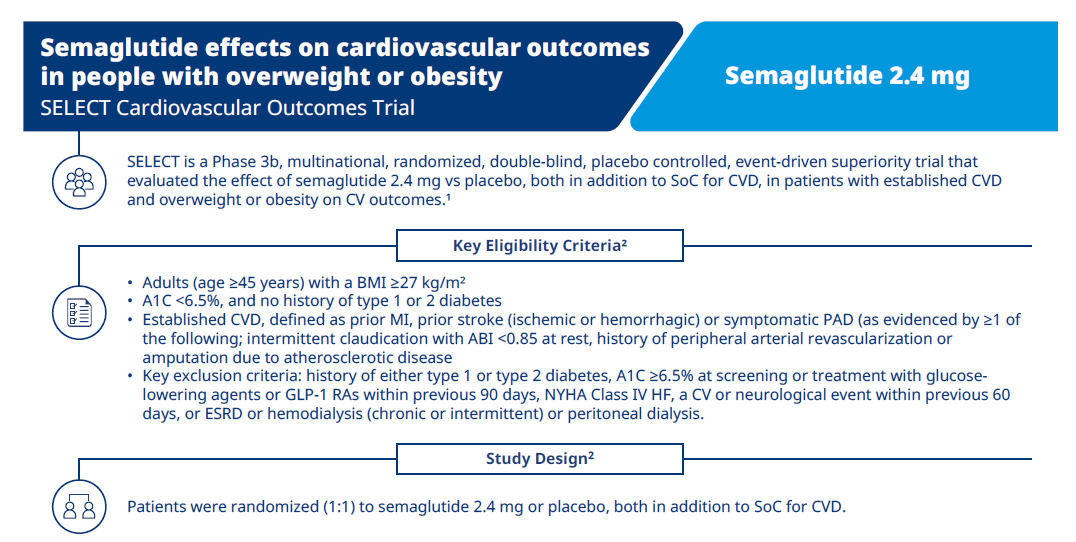
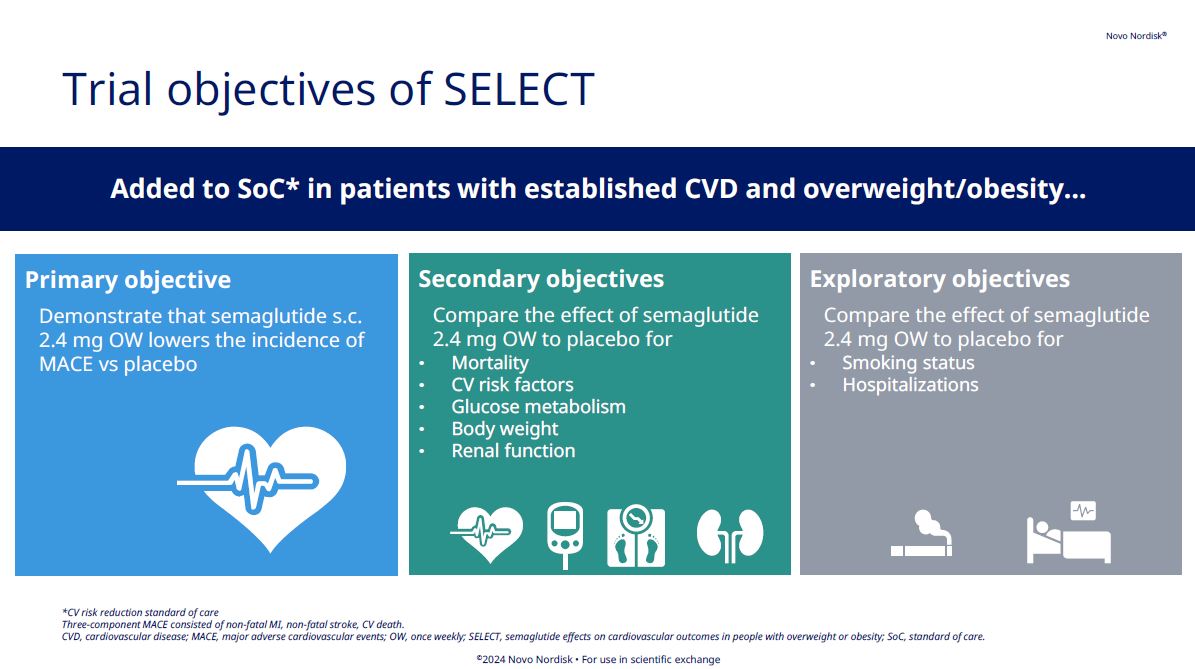
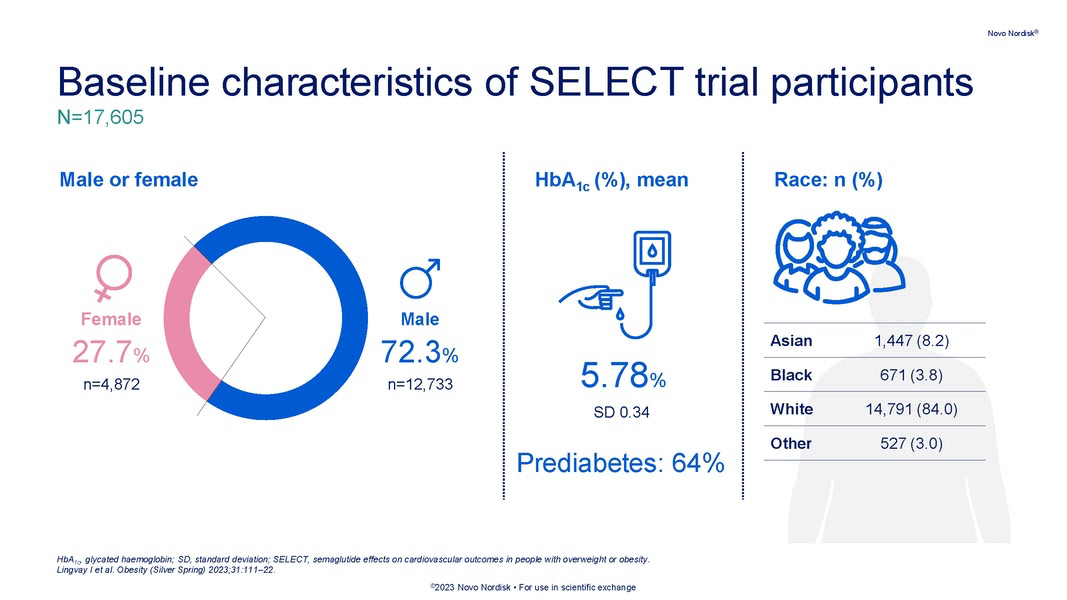
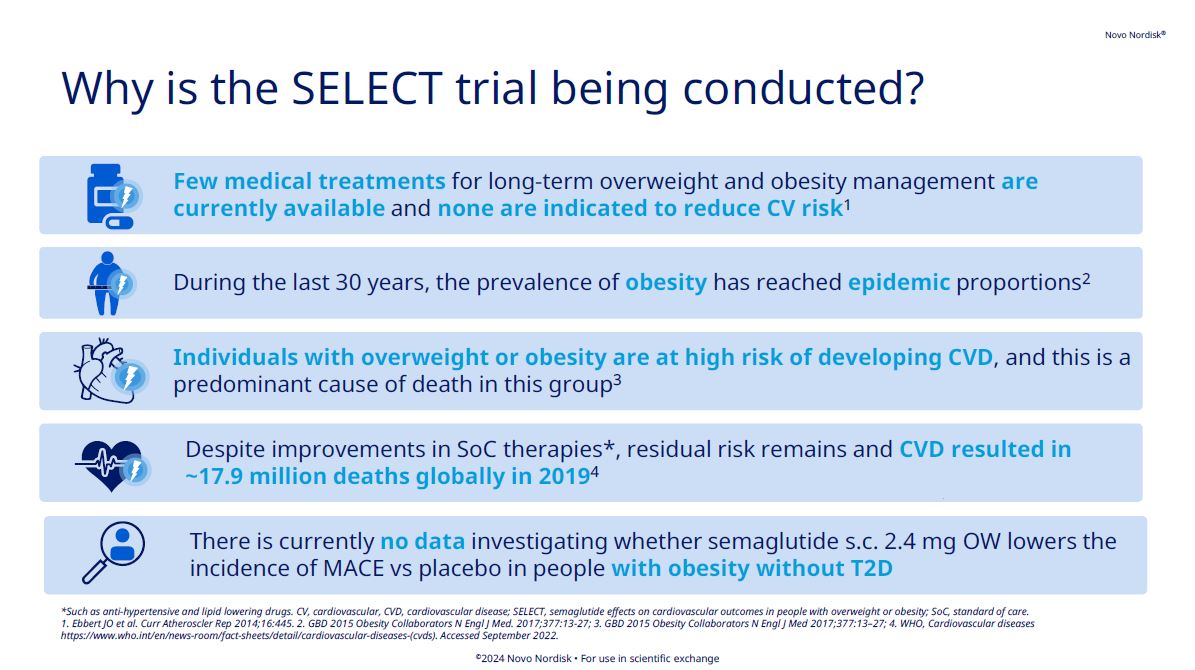
The double-blinded SELECT cardiovascular outcomes trial compared subcutaneous once-weekly semaglutide 2.4 mg with placebo as an adjunct to standard of care for prevention of major adverse cardiovascular (MACE) events over a period of up to 5 years. Read more about its background, methodology, results, and more.
Find more information
Speak to a medical representative
If you are a healthcare provider from the United States, click below to request information directly from the Novo Nordisk Medical Information department. If you are a patient, please visit here to contact us, or call 1-800-727-6500.
Search the Medical Information Database
The Scientific Exchange is a resource for U.S. Healthcare Professionals to learn more about disease states investigated by Novo Nordisk and our related products.